Pfanstiehl Sodium Succinate Citation Page
Menu
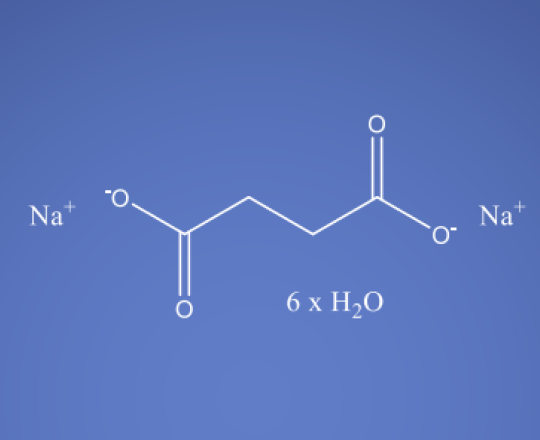
Sodium Succinate (Hexahydrate)
NF JPE
Molecular Formula
C4H16Na2O10
CAS No
Molecular Weight
Solubility in Water
Boiling Point
Melting Point
Quality Level
Highest – Parenteral Excipient Grade cGMP
Suitable for Injectable Biologic & Vaccine Formulations
Sodium Succinate hexahydrate is the sodium salt of succinic acid and commonly occurs as a hexahydrate, meaning it contains six water molecules in its crystal structure. Succinic acid is a dicarboxylic acid that is involved in various biochemical processes in the body.
Sodium succinate can act as a buffering agent, helping to maintain a stable pH in pharmaceutical formulations. This is crucial for the stability and efficacy of many drugs.
It can be used as a stabilizing agent in certain pharmaceutical formulations, preventing degradation or decomposition of active ingredients over time. Stabilizers are essential for ensuring the quality and shelf-life of pharmaceutical products.
Sodium succinate may improve the solubility of certain drugs, making them easier to formulate and administer. This is particularly important for drugs that have poor water solubility.
Sodium succinate hexahydrate may be incorporated into drug delivery systems, such as controlled-release formulations, to modulate the release of active ingredients over time.
Succinic acid, the parent compound of sodium succinate, has antioxidant properties. Antioxidants can help protect pharmaceutical formulations from oxidative degradation, preserving the integrity of the drugs.
Succinic acid is a naturally occurring component in the citric acid cycle, a central metabolic pathway in cells. It plays a role in energy production and various cellular processes. While the hexahydrate form may not directly impact cellular metabolism, the incorporation of succinate in pharmaceutical formulations could potentially influence certain biological processes.
Pfanstiehl Sodium Succinate Citation Page
Pfanstiehl Brand High Purity – Low Endotoxin – Low Metals Sodium Succinate (Hexahydrate)
was developed specifically for biopharmaceutical manufacturing and is produced under full ICH-Q7 cGMP conditions in the United States to the highest quality and purity standards.
Pfanstiehl Brand Sodium Succinate (Hexahydrate) is compliant with NF and JPE pharmacopoeia.
Because of these stringent manufacturing specifications and quality systems, you can be assured that Pfanstiehl’s Sodium Succinate (Hexahydrate) will be of the highest quality & consistency from batch to batch and the safest available for use in your manufacturing or research application.
Sodium Succinate Hexahydrate (Plant Derived)
Highest – Parenteral Excipient Grade GMP
Multi-Compendial Sodium Succinate (Hexahydrate)
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
NF
JPE
Upstream BioProcessing
Pharmaceutical Process
Biopharma Process
Pharmaceutical Final Formulation
Sodium Succinate Hexahydrate (Plant Derived)
Highest – Parenteral Excipient Grade GMP
Multi-Compendial Sodium Succinate (Hexahydrate)
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
NF
JPE
Upstream BioProcessing
Pharmaceutical Process
Biopharma Process
Pharmaceutical Final Formulation
100g, 1kg, 5kg, 10kg, 25kg, and 50kg