Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
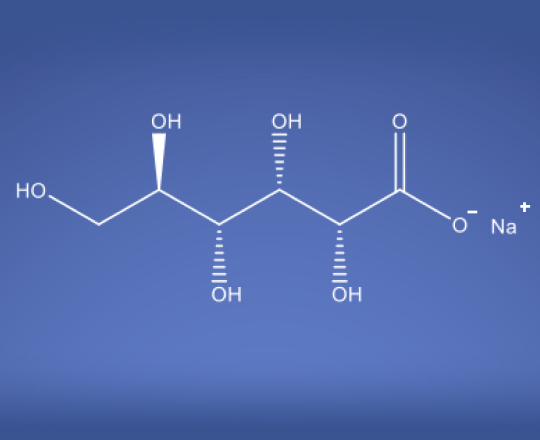
Sodium Gluconate USP
Molecular Formula
C6H12O7 • Na
CAS No
Molecular Weight
Solubility in Water
Boiling Point
Melting Point
Quality Level
Highest – Parenteral Excipient Grade cGMP
Suitable for Injectable Biologic & Vaccine Formulations
Large Volume Parenterals (LVP)
Sodium gluconate is a sodium salt of gluconic acid, which is derived from glucose through a simple fermentation process. It is a white crystalline powder that is highly soluble in water.
In Large Volume Parenterals, Sodium gluconate provides sodium ions (Na⁺), which are essential for maintaining electrolyte balance in the body. Sodium is one of the key electrolytes responsible for maintaining fluid balance, nerve function, and muscle contraction. In LVPs, it helps replenish sodium levels in the body, especially in situations where there is a deficit due to dehydration, electrolyte imbalances, or other medical conditions.
Sodium gluconate can effectively act as a buffering agent due to its ability to maintain a stable pH level in parenteral pharmaceutical formulations.
pH Regulation: Sodium gluconate possesses buffering capacity within a specific pH range, typically around pH 6 to 8, making it suitable for many pharmaceutical applications where this pH range is desirable.
pH Stability: It helps to resist changes in pH by neutralizing acidic or basic compounds that may be present in the formulation. This ensures that the pH remains within the desired range, which is crucial for maintaining the stability and efficacy of the pharmaceutical product.
Sodium gluconate is compatible with many other pharmaceutical ingredients commonly found in Large Volume Parenteral formulations. Its compatibility ensures that the solution remains homogeneous and does not precipitate or form insoluble complexes.
Sodium gluconate can be used as an excipient, a substance added to a pharmaceutical formulation to improve its properties. It can enhance the stability, solubility, and bioavailability of therapeutics.
Pfanstiehl Brand High Purity – Low Endotoxin – Low Metal Sodium Gluconate was developed specifically for Large Volume Parenterals, biopharmaceutical formulation,and commercial manufacturing and is produced under full ICH-Q7 cGMP conditions in the United States to the highest quality and purity standards.
Pfanstiehl Brand Sodium Gluconate is compliant with the USP monograph established in 2017 and available as USP (United States Pharmacopoeia)
Because of stringent quality systems and manufacturing specifications, you can be assured that Pfanstiehl’s Sodium Gluconate will be of the highest quality & consistency from batch to batch and the safest available for use in your manufacturing or research application.
Sodium Gluconate
Highest – Parenteral Excipient Grade GMP
Compendial Sodium Gluconate
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
USP
Large Volume Parenteral Formulation
Sodium Gluconate
Highest – Parenteral Excipient Grade GMP
Compendial Sodium Gluconate
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
USP
Large Volume Parenteral Formulation
100g, 1kg, 5kg, 10kg, 25kg, and 50kg