Pfanstiehl TRIS HCl Citation Page
Menu
TRIS HCl
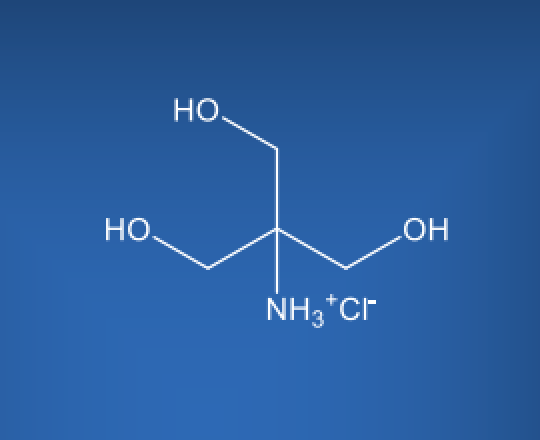
Molecular Formula
C4H12ClNO3
CAS No
Molecular Weight
Solubility in Water
Boiling Point
Melting Point
Quality Level
Highest – Parenteral Excipient Grade cGMP
Suitable for Injectable Biologic & Vaccine Formulations
TRIS HCl is a versatile buffering agent widely utilized in the pharmaceutical industry. This chemical compound plays a crucial role in maintaining a stable pH environment, which is vital for various biochemical and pharmaceutical processes.
TRIS HCl is a white crystalline powder that is highly soluble in water, making it easily incorporated into various pharmaceutical formulations. Its chemical structure consists of a trihydroxymethylaminomethane molecule combined with a hydrochloride group. TRIS HCl pKa is approximately 8.1 at 25°C, which makes it an effective buffer within the physiological pH range.
TRIS HCl pH range is 7.2 to 9.0 and is known for exceptional buffering capacity. This makes it ideal for applications where a stable pH environment is crucial, such as the manufacturing of pharmaceuticals. Maintaining the desired pH is essential for the stability and efficacy of drugs, as well as for the reproducibility of various biochemical processes.
TRIS HCl is considered biocompatible, meaning it is generally well-tolerated by living organisms. This characteristic is particularly important in pharmaceutical formulations intended for human use. The compatibility of TRIS HCl with biological systems makes it a preferred choice for buffering solutions in drug development.
TRIS HCl is versatile and can be used in various pharmaceutical applications, including protein and enzyme purification, cell culture, and nucleic acid isolation. Its compatibility with a wide range of substances makes it a valuable buffering component for pharmaceutical manufacturers working with diverse formulations.
The stability of TRIS HCl, both in solid form and in solution, contributes to its reliability as a buffering agent. Pharmaceutical products often undergo extended storage periods, and the stability of the buffering system becomes crucial in maintaining the integrity of the formulation over time.
TRIS HCl exhibits minimal interference with biochemical and enzymatic reactions. This property is vital in ensuring that the buffering component does not negatively impact the performance of the pharmaceutical product. The low reactivity of TRIS HCl enhances its applicability in various drug development processes.
TRIS HCl is a valuable buffering component in the pharmaceutical industry due to its excellent pH-regulating properties, biocompatibility, versatility, stability, and minimal interference with biochemical processes.
Pfanstiehl TRIS HCl Citation Page
Pfanstiehl’s TRIS HCl (CAS 1185-53-1) was developed specifically for biopharmaceutical formulation and commercial manufacturing and is produced under full ICH-Q7 cGMP conditions in the United States to the highest quality and purity standards.
Because of these stringent manufacturing specifications and Pfanstiehl quality systems, you can be assured that Pfanstiehl’s TRIS HCL will be of the highest quality & consistency from batch to batch and the safest available for use in your manufacturing or research application.
TRIS HCl (Synthetic)
Highest – Parenteral Excipient Grade GMP
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
Parenteral Formulation
Liquid Formulation
Pharmaceutical Process
Biopharma Process
Buffer Formulation
TRIS HCl (Synthetic)
Highest – Parenteral Excipient Grade GMP
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
Parenteral Formulation
Liquid Formulation
Pharmaceutical Process
Biopharma Process
Buffer Formulation
100g, 1kg, 5kg, 10kg, 25kg, and 50kg