Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
L-Glutamine USP JP ChP
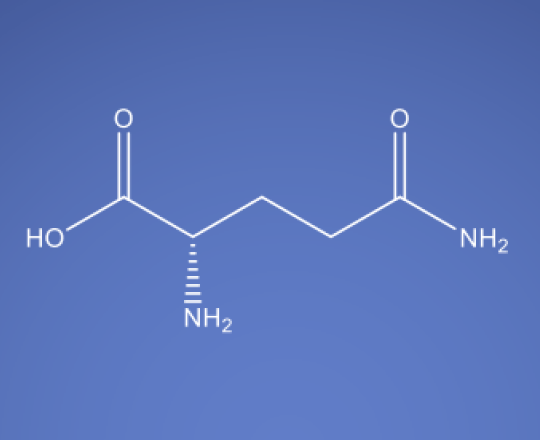
Molecular Formula
C6H14N4O2
CAS No
Molecular Weight
Solubility in Water
Boiling Point
Melting Point
Quality Level
Highest – Parenteral Excipient Grade cGMP
Multi-Compendial L-Glutamine
Suitable for Injectable Biologic & Vaccine Formulations
L-Glutamine is an essential amino acid involved in numerous physiological processes within mammalian cells. In upstream cell culture protein processes, L-Glutamine plays a pivotal role in supporting cell growth, viability, and protein production.
L-Glutamine serves as a primary energy source for rapidly proliferating cells. During cell culture, cells consume L-Glutamine as a substrate for energy production via aerobic glycolysis and the tricarboxylic acid (TCA) cycle. Adequate L-Glutamine availability ensures that cells have the energy necessary for cellular processes, including DNA replication, protein synthesis, and maintenance of cellular structures. Insufficient levels of L-Glutamine can lead to metabolic stress, impairing cell growth and viability, and ultimately compromising protein production.
L-Glutamine is a key component in protein synthesis. It provides the amino group required for the synthesis of other amino acids, serving as a precursor for the synthesis of proteins and nucleotides. In cell culture systems used for protein production, cells require abundant L-Glutamine to support the high demand for protein synthesis associated with recombinant protein expression. Optimizing L-Glutamine concentration in the culture medium is essential to maximize protein yield and ensure proper folding and post-translational modification of the expressed protein.
L-Glutamine plays a crucial role in osmoregulation, helping to maintain cellular osmotic balance and prevent osmotic stress. In cell culture, fluctuations in osmolarity can occur due to changes in media composition, cell metabolism, or environmental conditions. L-Glutamine acts as an osmolyte, regulating intracellular osmotic pressure and protecting cells from osmotic stress. Maintaining appropriate L-Glutamine levels in the culture medium is essential for preventing osmotic imbalances that could compromise cell health and protein production.
Beyond its roles as a metabolic substrate, L-Glutamine also functions as a signaling molecule involved in various cellular processes, including cell proliferation, differentiation, and apoptosis. L-Glutamine can modulate signaling pathways such as the mammalian target of rapamycin (mTOR) pathway, which regulates protein synthesis and cell growth. Additionally, L-Glutamine contributes to the synthesis of glutathione, a critical antioxidant molecule that helps protect cells from oxidative stress and maintain cellular redox balance.
The purity of L-Glutamine is critical to ensure that the cell culture environment is free from contaminants that could negatively impact cell growth and protein expression. Impurities in L-Glutamine could lead to cell stress or death, affecting protein yield and quality.
Consistency in L-Glutamine quality is essential for reproducibility in cell culture experiments and protein production processes. Fluctuations in L-Glutamine concentration or impurity levels can lead to variability in cell growth and protein expression, impacting the consistency and reliability of experimental results.
The quality of L-Glutamine directly impacts cell health and viability. Poor-quality L-Glutamine may contain toxic contaminants or degradation products that can impair cell growth and protein production, leading to lower yields and inferior protein quality.
Pfanstiehl Brand High Purity – Low Endotoxin – Low Metals L-Glutamine was developed specifically for biopharmaceutical manufacturing and is produced under full ICH-Q7 cGMP conditions in the United States to the highest quality and purity standards.
Pfanstiehl Brand Multi-Compendial L-Glutamine is compliant with the USP, JP (Japan) and ChP (China) Pharmacopeia.
Because of these stringent manufacturing specifications and quality systems, you can be assured that Pfanstiehl’s L-Glutamine will be of the highest quality & consistency from batch to batch and the safest available for use in your upstream cell culture applications.
L-Glutamine (Plant Derived)
Highest – Parenteral Excipient Grade GMP
Multi-Compendial L-Glutamine
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
USP
JP
ChP
Parenteral Formulation
Liquid Formulation
Pharmaceutical Process
Biopharma Process
Upstream Protein Production
Upstream Feeds
L-Glutamine (Plant Derived)
Highest – Parenteral Excipient Grade GMP
Multi-Compendial L-Glutamine
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
USP
JP
ChP
Parenteral Formulation
Liquid Formulation
Pharmaceutical Process
Biopharma Process
Upstream Protein Production
Upstream Feeds
100g, 1kg, 5kg, 10kg, 25kg, and 50kg