Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
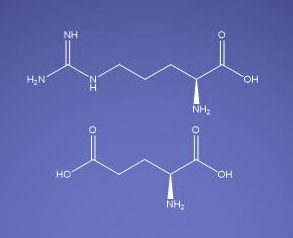
Molecular Formula
C11H23N5O6
CAS No
Molecular Weight
Solubility in Water
Boiling Point
Melting Point
Quality Level
Highest – Parenteral Excipient Grade GMP
Arginine glutamate is a compound composed of two naturally occurring amino acids: arginine and glutamic acid. These amino acids are combined to form a salt, known as an amino acid ionic pair, which is widely used in pharmaceuticals, especially as an excipient. Arginine glutamate is particularly valued in the formulation of parenteral (injectable) therapeutics due to its unique chemical and biological properties.
The effectiveness of arginine glutamate as an excipient stems from several key properties that make it ideal for parenteral applications:
Pfanstiehl Arginine glutamate is an excellent excipient option for parenteral injectable therapeutics due to its ability to enhance solubility, stabilize proteins, maintain pH, and prevent aggregation. Its biocompatibility, low toxicity, and versatility across various therapeutic modalities make it a valuable tool in pharmaceutical formulations.
Pfanstiehl Brand High Purity – Low Endotoxin – Low Metals
Arginine Glutamate
was developed specifically for biopharmaceutical manufacturing and is produced under full ICH-Q7 cGMP conditions in the United States to the highest quality and purity standards.
Because of these stringent manufacturing specifications and Pfanstiehl quality systems, you can be assured that Pfanstiehl’s Arginine Glutamate will be of the highest quality & consistency from batch to batch and the safest available for use in your manufacturing or research application.
Arginine Glutamate
Highest Quality
ICH-Q7 cGMP
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
No active monographs exist
Pfanstiehl is sponsoring the USP monograph
Parenteral Formulation
Liquid Formulation
Pharmaceutical Process
Biopharma Process
Arginine Glutamate
Highest Quality
ICH-Q7 cGMP
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
No active monographs exist
Pfanstiehl is sponsoring the USP monograph
Parenteral Formulation
Liquid Formulation
Pharmaceutical Process
Biopharma Process
100g, 1kg, 5kg, 10kg, 25kg, and 50kg