Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
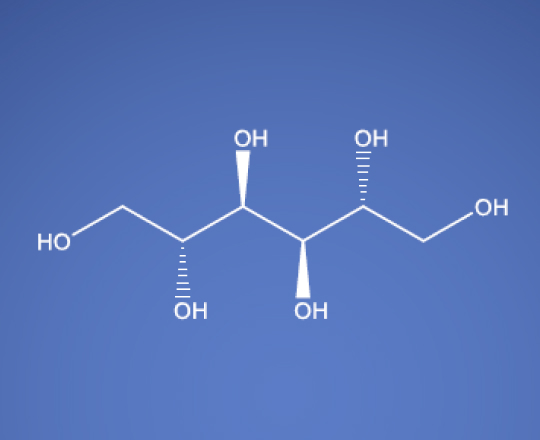
D-Mannitol USP EP BP JP ChP
Molecular Formula
C6H14O6
CAS No
Molecular Weight
Solubility in Water
Boiling Point
Melting Point
Quality Level
Highest – Parenteral Excipient Grade GMP
Multi-Compendial D-Mannitol
Suitable for Injectable Biologic & Vaccine Formulations
D-Mannitol is a type of sugar alcohol that is commonly used in the pharmaceutical and food industries. It is a white, crystalline powder that is naturally occurring and can be found in fruits, vegetables, and certain plants. Pfanstiehl Multi-Compendial D-Mannitol is often used as an excipient in pharmaceutical formulations, including biologics, due to its various beneficial properties.
D-Mannitol is known to stabilize proteins in both liquid and lyophilized (freeze-dried) formulations. It helps to prevent protein aggregation and denaturation, which can occur during manufacturing and storage.
In freeze-drying processes, Pfanstiehl D-Mannitol acts as a cryoprotectant and lyoprotectant. It protects the biological molecules from damage caused by freezing and drying stresses, ensuring the integrity and activity of the biologic product.
D-Mannitol is an effective osmotic agent, which helps in controlling the osmotic pressure in formulations. This is particularly important in maintaining the stability and efficacy of biologics during storage and administration.
D-Mannitol serves as a bulking agent in lyophilized formulations, providing structure and ensuring that the final product has a uniform and acceptable appearance.
Pfanstiehl D-Mannitol is highly soluble in water, making it easy to formulate into liquid preparations. It is also compatible with a wide range of active pharmaceutical ingredients (APIs) and other excipients, enhancing its versatility in different formulations.
D-Mannitol is generally recognized as safe (GRAS) by regulatory authorities like the FDA. It is well-tolerated by patients, with minimal side effects when used in appropriate amounts.
Pfanstiehl Brand High Purity Low Endotoxin Low Metals (HPLE-LM)TM Multi-Compendial D-Mannitol was developed specifically for biopharmaceutical use and is produced under full cGMP conditions in the United States to the highest quality and purity standards.
Because of stringent quality systems and manufacturing specifications, you can be assured that Pfanstiehl’s (HPLE-LM)TM D-Mannitol will be of the highest quality & consistency from batch to batch and the safest product available for use in your manufacturing or research application.
D-Mannitol
Highest – Parenteral Excipient Grade GMP
Multi-Compendial D- Mannitol
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
USP
EP
BP
JP
ChP
Parenteral Formulation
Lyophilization
Cryoprotection
Spray-Drying
D-Mannitol
Highest – Parenteral Excipient Grade GMP
Multi-Compendial D- Mannitol
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
USP
EP
BP
JP
ChP
Parenteral Formulation
Lyophilization
Cryoprotection
Spray-Drying
100g, 1kg, 5kg, 10kg, 25kg, and 50kg