Pfanstiehl Maltose Monohydrate Citation Page
Menu
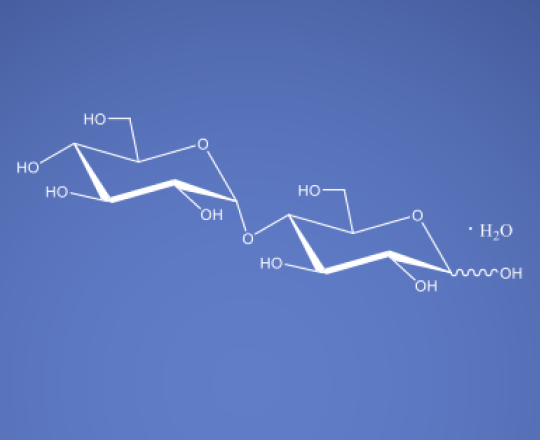
Maltose Monohydrate NF JP ChP
Molecular Formula
C12H22O11 · H2O
CAS No
Molecular Weight
Solubility in Water
Boiling Point
Melting Point
Quality Level
Highest – Parenteral Excipient Grade cGMP
Multi-Compendial Maltose monohydrate
Suitable for Injectable Biologic & Vaccine formulation
Maltose monohydrate is a disaccharide sugar composed of two glucose molecules linked together through an α(1→4) glycosidic bond. It is a carbohydrate commonly found in various food sources, including malted grains, such as barley, and is often used in the food industry as a sweetener and flavor enhancer. Beyond its role in the culinary world, maltose has found applications in the field of medicine, particularly in stabilizing infusions, blood fractionation, and preventing protein aggregation in Intravenous Immunoglobulin (IVIG) solutions.
One of the key benefits of using Maltose in medical applications is its ability to act as a stabilizing agent in infusions. Infusions, which involve the administration of fluids or drugs directly into the bloodstream, are crucial in various medical treatments. Maltose, with its chemical properties, aids in maintaining the stability of certain drugs and compounds during the infusion process. Its inclusion in infusion solutions helps prevent the degradation or denaturation of therapeutic agents, ensuring that the desired effects are delivered to the patient effectively.
Blood fractionation is another area where maltose plays a significant role. Blood fractionation is the process of separating blood components, such as plasma, red blood cells, and platelets. Maltose is often used in the stabilization of blood-derived products, contributing to the preservation of their biological activity. The inclusion of maltose in the fractionation process helps maintain the integrity of blood components, ensuring that they can be safely stored and administered for various medical purposes.
Maltose monohydrate is a valuable additive for preventing protein aggregation. IVIG is a therapeutic treatment involving the infusion of immunoglobulins to boost the immune system. Protein aggregation, where proteins clump together, can lead to reduced efficacy and potential adverse effects. Maltose, with its stabilizing properties, helps prevent such aggregation, ensuring that the immunoglobulins maintain their structural integrity and therapeutic effectiveness.
The prevention of protein aggregation in IVIG solutions is particularly crucial as it directly impacts the safety and efficacy of the treatment. Maltose acts as a protective agent, maintaining the solubility and stability of proteins in the solution. This contributes to the overall success of IVIG therapy by ensuring that patients receive a consistent and reliable dose of immunoglobulins without the risk of adverse reactions.
Pfanstiehl Maltose Monohydrate Citation Page
Pfanstiehl’s High Purity, Low Endotoxin, and Low Metals Maltose Monohydrate is manufactured under ICH-Q7 cGMP conditions and in compliance with the USP-NF JP Chp Pharmacopeia.
All Pfanstiehl products are made in the USA.
Because of these stringent manufacturing specifications and quality systems, you can be assured that Pfanstiehl’s Multi-Compendial Maltose monohydrate will be of the highest consistency from batch to batch and the safest available for use in your drug product formulation or research application.
Maltose Monohydrate (Plant Derived)
Highest – Parenteral Excipient Grade GMP
Multi-Compendial Maltose monohydrate
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
NF
JP
Chp
Parenteral Formulation
Liquid Formulation
Pharmaceutical Process
Biopharma Process
Aggregation Prevention in Intravenous Immunoglobulin Solutions (IVIG)
Maltose Monohydrate (Plant Derived)
Highest – Parenteral Excipient Grade GMP
Multi-Compendial Maltose monohydrate
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
NF
JP
Chp
Parenteral Formulation
Liquid Formulation
Pharmaceutical Process
Biopharma Process
Aggregation Prevention in Intravenous Immunoglobulin Solutions (IVIG)
100g, 1kg, 5kg, 10kg, 25kg, and 50kg