Pfanstiehl Trehalose dihydrate Citation Page
Menu
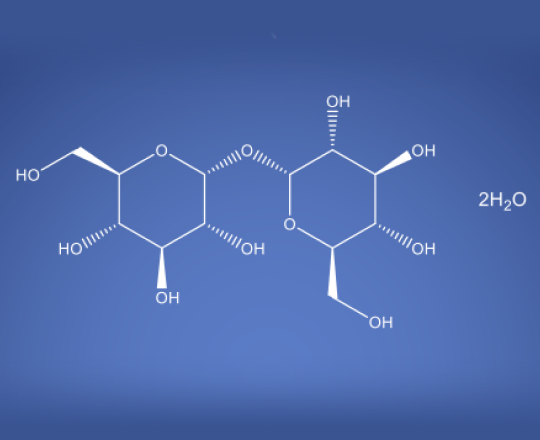
Trehalose Dihydrate NF EP BP JP ChP
Molecular Formula
C12 H22O11 . 2H2O
CAS No
Molecular Weight
Solubility in Water
Boiling Point
Melting Point
Quality Level
Trehalose dihydrate is a sugar made of two glucose units connected by a strong α-1,1 bond making it stable in acidic and high-temperature conditions. Unlike other sugars it does not undergo glycation because its structure keeps the glucose molecules in a closed-ring form. The carbohydrate is less soluble than sucrose but more soluble at temperatures above 80°C. It’s stability and resistance to breakdown make it useful in biological preservation because of its cryoprotective properties.
Trehalose excipient grade has a high glass transition temperature (Tg) which is typically around 115°C when it is anhydrous. The glass transition temperature is when a substance changes from a rubbery flexible state to a solid glass-like state. This property is helpful in preserving protein therapeutics by trapping proteins in a stable glassy form which helps prevent degradation.
Below the Transition Glass Temperature (Tg), molecules in the glassy state have minimal movement. This minimizes the risk of proteins aggregating or denaturing which can harm the product’s effectiveness. Trehalose is ideal for protecting sensitive biologics during freeze-drying (lyophilization) and storage and helps preserve protein structure and function for therapeutic use.
Trehalose Excipient is a high-purity, low endotoxin, low metals excipient that meets the stringent quality and regulatory requirements outlined in multiple pharmacopeias (e.g., USP, EP, BP, JP, ChP). It is specifically manufactured and tested to ensure it is suitable for use in parenteral (injectable) drug products, where safety, sterility, and low impurity levels are critical.
Low Endotoxin Trehalose excipient is used in the biopharmaceutical industry to stabilize proteins, lipids, and carbohydrates throughout the formulation and freeze/thaw life cycle of therapeutics. It is also widely applied as a cryopreservative and media supplement in a variety of cell-based bioprocesses.
Pfanstiehl Trehalose dihydrate Citation Page
Trehalose dihydrate (Plant derived)
Trehalose dihydrate (Plant derived)