Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
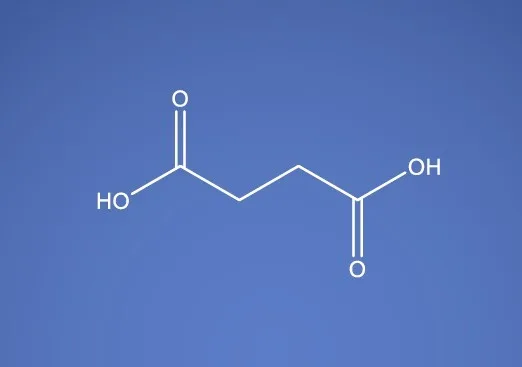
Succinic Acid USP/NF EP BP JP ChP
Molecular Formula
C4H6O4
CAS No
Molecular Weight
Solubility in Water
Boiling Point
Melting Point
Quality Level
Highest – Parenteral Excipient Grade cGMP
Suitable for Injectable Biologic & Vaccine Formulations
Succinic acid is a dicarboxylic acid with the chemical formula C₄H₆O₄. It naturally occurs in various plants and animals and is an important intermediate in the citric acid cycle (Krebs cycle), which is a key metabolic pathway that generates energy in cells.
In Biologic formulations, Succinic acid’s role is to ensure the stability, efficacy, and safety of the biologic product throughout its shelf life and during administration.
Succinic acid’s buffering capacity is a key factor in its widespread use in biologic formulations. By maintaining a stable pH environment, succinic acid helps ensure the stability, efficacy, and shelf life of biologic products. Its broad buffering range, compatibility with biologic molecules, and synergy with other excipients make it an invaluable component in the formulation of stable and effective biologic therapeutics.
Pfanstiehl Brand High Purity – Low Endotoxin – Low Metals
Succinic Acid was developed specifically for biopharmaceutical formulation and commercial manufacturing and are produced under full ICH-Q7 cGMP conditions in the United States to the highest quality and purity standards.
Pfanstiehl Brand Succinic Acid is compliant with NF (National Formulary) EP (European) BP (United Kingdom), JP (Japanese), and ChP (Chinese) Pharmacopoeia
Because of these stringent manufacturing specifications and Pfanstiehl Quality Systems, you can be assured that Pfanstiehl’s Succinic Acid will be of the highest quality & consistency from batch to batch and the safest available for use in your manufacturing or research application.
Succinic Acid
Highest – Parenteral Excipient Grade GMP
Multi-Compendial Succinic Acid
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
USP/NF
EP
BP
JP
ChP
Parenteral Formulation
Succinic Acid
Highest – Parenteral Excipient Grade GMP
Multi-Compendial Succinic Acid
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
USP/NF
EP
BP
JP
ChP
Parenteral Formulation
100g, 1kg, 5kg, 10kg, 25kg, and 50kg