Menu
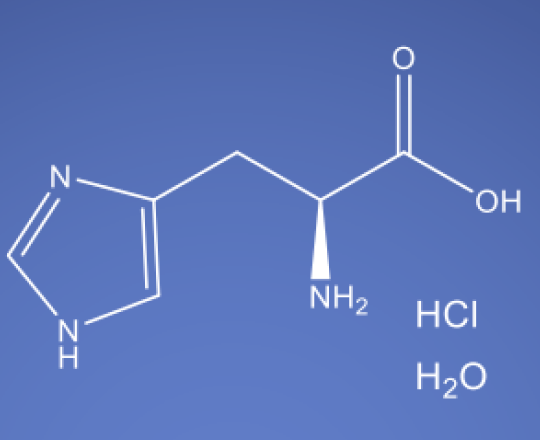
Molecular Formula
C6H10ClN3O2• H2O
CAS No
Molecular Weight
Solubility in Water
Boiling Point
Melting Point
Quality Level
Highest – Parenteral Excipient Grade cGMP
Suitable for Injectable Biologic & Vaccine Formulations
Drug Master File (DMF) # CBER 27258
Chinese CDE # F20200000337
L-Histidine HCl Excipient GMP , the salt of an essential amino acid, plays a crucial role in various biological processes within the human body. Beyond its physiological functions, L-Histidine HCl has garnered attention in the pharmaceutical industry for its unique ability to enhance the stability of monoclonal antibodies (mAbs) during the manufacturing processes.
Monoclonal antibodies and other protein based biologic therapeutics are inherently susceptible to degradation, aggregation, and loss of activity during manufacturing and storage. L-Histidine HCl emerges as a key player in addressing these challenges due to its unique chemical properties.
L-Histidine HCl Excipient GMP exhibits an excellent buffering capacity, particularly at pH 7.4, making it an ideal amino acid for maintaining a stable environment during the production and storage of mAbs. The pH level is a critical factor influencing the stability and solubility of proteins. By utilizing L-Histidine at pH 7.4, manufacturers create an environment that closely mimics the physiological conditions within the human body, ensuring the stability of mAbs.
L-Histidine HCl Excipient GMP possesses metal-chelating properties, which can be advantageous in preventing metal-catalyzed oxidation and degradation of mAbs. Metal ions such as copper and iron can accelerate oxidative reactions, leading to the formation of reactive oxygen species. By chelating these ions, the amino acid acts as a protective agent, mitigating the risk of oxidative damage to mAbs.
The solubility of proteins is a critical parameter during manufacturing and formulation processes. L-Histidine HCl, especially at pH 7.4, aids in maintaining the solubility of mAbs, preventing aggregation and precipitation that could compromise the efficacy of the final product.
Pfanstiehl Brand High Purity – Low Endotoxin – Low Metals
L-Histidine Hydrochloride BP EP JP ChP
L-Histidine Hydrochloride Inhalation Grade BP EP JP ChP
were developed specifically for biopharmaceutical manufacturing and are produced under full ICH-Q7 cGMP conditions in the United States to the highest quality and purity standards.
Pfanstiehl Brand Amino Acids are compliant with multiple pharmacopoeia.
Because of these stringent manufacturing specifications and Pfanstiehl quality systems, you can be assured that Pfanstiehl products will be of the highest quality & consistency from batch to batch and the safest available for use in your manufacturing or research application.
L-Histidine HCl (Plant Derived)
Highest – Compendial Parenteral Excipient Grade GMP
Drug Master File (DMF) # CBER 27258
Chinese CDE # F20200000337
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
BP
EP
JP
ChP
Parenteral Formulation
Liquid Formulation
Pharmaceutical Process
Biopharma Process
Pharmaceutical Buffering
L-Histidine HCl (Plant Derived)
Highest – Compendial Parenteral Excipient Grade GMP
Drug Master File (DMF) # CBER 27258
Chinese CDE # F20200000337
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
BP
EP
JP
ChP
Parenteral Formulation
Liquid Formulation
Pharmaceutical Process
Biopharma Process
Pharmaceutical Buffering
100g, 1kg, 5kg, 10kg, 25kg, and 50kg