Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
Lowest Nitrosamines
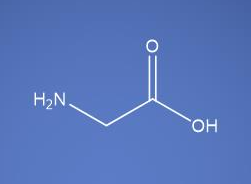
Glycine USP EP BP JP ChP
Molecular Formula
C2H5NO2
CAS No
Molecular Weight
Solubility in Water
Boiling Point
Melting Point
Quality Level
Highest – Parenteral Excipient Grade cGMP
Suitable for Injectable Biologic & Vaccine Formulations
Glycine is the simplest and only achiral amino acid among the proteinogenic amino acids. It is characterized by a hydrogen atom as its side chain, making it distinct in structure and function. As a non-essential amino acid, glycine plays a fundamental role in various biological processes, including protein synthesis, cellular function, and the production of critical compounds such as creatine and heme. Classified as an alpha-amino acid and part of the serine family, glycine is non-polar, non-optical, and glucogenic. Additionally, it exists in equilibrium with its zwitterionic form, a feature that contributes to its versatility in biological systems.
Due to its structural simplicity and biological relevance, glycine is an important component and precursor for many macromolecules in cells. Its unique properties make it highly valuable in various applications, particularly in the formulation and production of biologic therapeutics. In the pharmaceutical industry, glycine is frequently used as an excipient, an inactive substance included in medications alongside the active ingredient. Its inclusion as an excipient in the final formulation of biologic therapeutics offers several significant benefits.
One of the primary advantages of using glycine in biologic therapeutics is its ability to stabilize protein-based drugs, known as biologics. During formulation, storage, and administration, protein aggregation, denaturation, and degradation can compromise the efficacy of biologics. Glycine helps to mitigate these issues by providing structural support to the protein molecules, thus maintaining their integrity and prolonging their shelf life. This stabilization is critical in ensuring the therapeutic effectiveness of biologic drugs.
Glycine also serves as an effective buffering agent in biologic formulations. Maintaining an appropriate pH is essential for preserving the stability and functionality of biologics, which are often sensitive to changes in their chemical environment. Glycine’s buffering capacity helps maintain the pH within an optimal range, ensuring that the biologic therapeutic remains stable and functional throughout its shelf life and administration.
In biologic therapeutics that undergo lyophilization (freeze-drying), glycine plays a crucial role in enhancing the stability and structure of the final product. It helps improve the formation of a solid cake during freeze-drying, ensuring that the biologic remains intact and well-preserved after reconstitution. This is particularly important for biologics that require long-term storage in a dried form before being reconstituted for use.
For biologic drugs administered via injection, it is essential that the formulation has a suitable osmolality to avoid irritation or damage to tissues and cells. Glycine acts as an osmolality adjuster, helping to create an isotonic solution that is more compatible with the body’s natural osmotic conditions. This makes the administration of biologics more comfortable for patients and reduces the risk of adverse effects at the injection site.
In formulation and storage processes that involve freezing, glycine serves as a cryoprotectant. During the freezing and thawing stages, biologic drugs can suffer from freezing-related damage, which can impair their efficacy. Glycine helps protect the biologics from such damage by preserving their structural integrity during the freezing process, ensuring that they remain stable and effective upon thawing.
Pfanstiehl Brand High Purity – Low Endotoxin – Low Metals
was developed specifically for biopharmaceutical manufacturing and is produced under full ICH-Q7 cGMP conditions in the United States to the highest quality and purity standards.
Pfanstiehl Brand Multi-Compendial Glycine is compliant with multiple pharmacopoeia.
Because of these stringent manufacturing specifications and Pfanstiehl Quality Systems, you can be assured that Pfanstiehl Glycine will be of the highest quality & consistency from batch to batch and the safest available for use in your manufacturing or research application.
Glycine (Synthetically Derived)
Highest – Parenteral Excipient Grade GMP
Multi-compendial Glycine
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
Lowest Nitrosamines
USP
EP
BP
JP
ChP
Parenteral Formulation
Liquid Formulation
Pharmaceutical Process
Biopharma Process
Glycine (Synthetically Derived)
Highest – Parenteral Excipient Grade GMP
Multi-compendial Glycine
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
Lowest Nitrosamines
USP
EP
BP
JP
ChP
Parenteral Formulation
Liquid Formulation
Pharmaceutical Process
Biopharma Process
100g, 1kg, 5kg, 10kg, 25kg, and 50kg