Pfanstiehl D-Galactose Citation Page
Menu
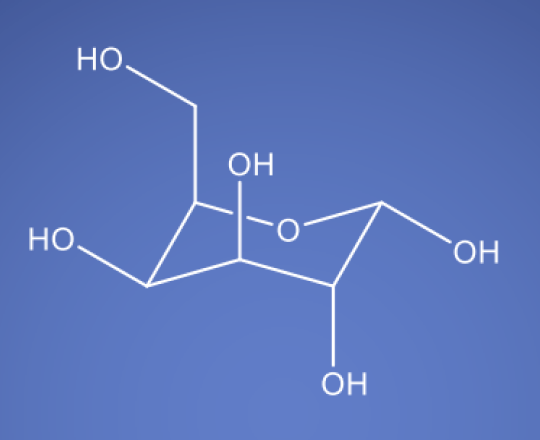
D-Galactose NF EP
Molecular Formula
C6H12O6
CAS No
Molecular Weight
Solubility in Water
Boiling Point
Melting Point
Quality Level
Highest – Parenteral Excipient Grade cGMP
Multi-Compendial D-Galactose
Suitable for Upstream Biologic Production Feeds
D-Galactose is a six-carbon sugar closely related to glucose, and it is a fundamental component of various glycolipids and glycoproteins. In bioprocessing, galactose has gained prominence due to its unique metabolic effects and its impact on cell culture conditions.
One of the primary challenges in large-scale cell culture for biologic protein production is the accumulation of lactate, a byproduct of anaerobic metabolism. Elevated levels of lactate can lead to a decrease in pH, causing stress to the cells and negatively affecting protein production. Galactose, when used as a carbon source, promotes oxidative metabolism, reducing the reliance on anaerobic pathways. This shift helps in minimizing lactate accumulation, maintaining optimal pH levels, and creating a more favorable environment for cell growth and protein synthesis.
Ammonia, another byproduct of cellular metabolism, can be detrimental to cell viability and protein quality in bioprocessing. Excessive ammonia levels can lead to toxicity, affecting cell health and compromising the integrity of the produced proteins. D-Galactose metabolism has been shown to reduce ammonia production compared to other carbon sources, offering a more controlled and stable environment for cell cultures. This reduction in ammonia levels contributes to enhanced cell viability and overall protein production efficiency.
By addressing the issues of lactate and ammonia accumulation, the use of galactose in biologic protein production ultimately leads to increased target protein yields. The optimized culture conditions provided by galactose as a carbon source create a more hospitable environment for cells to thrive, leading to improved cell growth and enhanced productivity. This, in turn, results in higher yields of the desired recombinant proteins, making galactose a valuable asset in the field of bioprocessing.
Supplementation of Pfanstiehl Multi-Compendial D-Galactose in cell culture media can help to modulate Galactosylation and Sialylation in recombinant Therapeutic glycoproteins. This may be particularly important during Biosimilars or Biobetters manufacturing where matching Glycosylation profile with innovator drugs or having improved efficacy than the innovator drugs is important. In some instances increasing therapeutic protein productivity can lead to decreased Galactosylation/Sialylation which can be mitigated by supplementing cell culture media with Galactose.
Pfanstiehl D-Galactose Citation Page
Pfanstiehl Brand High Purity – Low Endotoxin – Low Metals D-Galactose was developed specifically for biopharmaceutical manufacturing and is produced under full ICH-Q7 cGMP conditions in the United States to the highest quality and purity standards.
Pfanstiehl Brand D-Galactose is compliant with the NF (National Formulary) and EP (European) Pharmacopeia.
Because of these stringent manufacturing specifications and quality systems, you can be assured that Pfanstiehl’s Galactose will be of the highest quality & consistency from batch to batch and the safest available for use in your upstream cell culture applications
D-Galactose (Plant Derived)
Highest – Parenteral Excipient Grade GMP
Multi-Compendial D-Galactose
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
NF
EP
Parenteral Formulation
Liquid Formulation
Pharmaceutical Process
Biopharma Process
D-Galactose (Plant Derived)
Highest – Parenteral Excipient Grade GMP
Multi-Compendial D-Galactose
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
NF
EP
Parenteral Formulation
Liquid Formulation
Pharmaceutical Process
Biopharma Process
100g, 1kg, 5kg, 10kg, 25kg, and 50kg