Pfanstiehl L-Arginine Citation Page
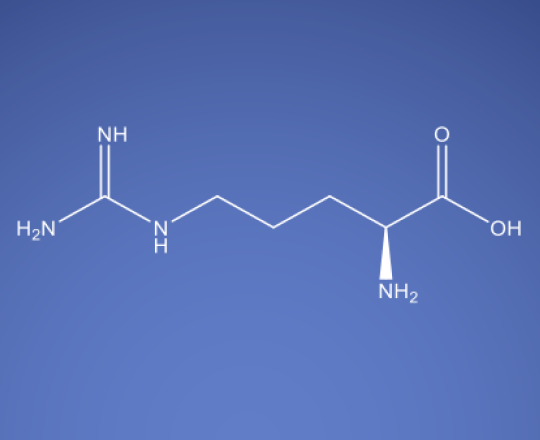
Molecular Formula
C6H14N4O2
CAS No
Molecular Weight
Solubility in Water
Boiling Point
Melting Point
Quality Level
Highest – Parenteral Excipient Grade cGMP
Suitable for Injectable Biologic & Vaccine Formulations
Drug Master File (DMF) # CBER 19116 & CDER 38012
Chinese CDE # F20190000412
L-Arginine is an essential amino acid that is physiologically active in the L- form. It plays various roles in the body, including its involvement in protein synthesis. In the context of stabilizing proteins in liquid and lyophilization processes, L-Arginine has been studied for its potential to protect and stabilize proteins during these processes.
Lyophilization, also known as freeze-drying, is a process used to remove water from proteins and other biological materials, allowing for their long-term storage and preservation. However, the freeze-drying process can pose challenges to the stability of proteins, as the freezing and drying steps can lead to denaturation or aggregation of the protein molecules.
L-Arginine has been investigated for its ability to mitigate these challenges and provide stabilization during lyophilization.
Pfanstiehl L-Arginine has been shown to reduce protein aggregation during freezing and drying processes. It helps maintain the native conformation of proteins and prevents them from sticking together, which is crucial for maintaining their biological activity.
L-Arginine can enhance the solubility of proteins in solution, even at low temperatures. This can be advantageous during the freezing step of lyophilization, where maintaining protein solubility is important for preventing aggregation.
Pfanstiehl Multi-Compendial L-Arginine is known for its role in stabilizing protein structures. It can interact with proteins through various mechanisms, including hydrogen bonding and charge interactions, helping to preserve the native structure of proteins.
L-Arginine has also been studied for its ability to influence ice crystal formation during freezing. By modifying the freezing process, it may help reduce damage to proteins caused by the formation of ice crystals.
Pfanstiehl Multi-Compendial L-Arginine plays a crucial role in mitigating viscosity challenges in high-concentration protein formulations. High protein concentrations often result in increased solution viscosity, which can impede manufacturing processes and affect the administration of therapeutic proteins. L-Arginine’s influence on viscosity reduction is attributed to its role as a solubilizing agent and its ability to disrupt protein-protein interactions. The positively charged guanidinium group of L-Arginine interacts with negatively charged protein surfaces, preventing the formation of aggregates and reducing the overall viscosity of the formulation. This property enhances the flowability of high-concentration protein solutions, facilitating their handling during manufacturing and administration processes. The dual functionality of L-Arginine in both stabilizing proteins and reducing viscosity makes it a versatile and valuable additive in the formulation of high-concentration protein-based products.
Pfanstiehl L-Arginine Citation Page
Pfanstiehl Brand High Purity – Low Endotoxin – Low Metals
L-Arginine USP EP JP ChP
developed specifically for biopharmaceutical manufacturing and produced under full ICH-Q7 cGMP conditions in the United States to the highest quality and purity standards.
Pfanstiehl Brand L-Arginine are compliant with USP EP JP and ChP pharmocopoeia.
Because of these stringent manufacturing specifications and quality systems, you can be assured that Pfanstiehl’s L-Arginine will be of the highest quality & consistency from batch to batch and the safest available for use in your manufacturing or research application.
L-Arginine (Plant Derived)
Highest – Parenteral Excipient Grade GMP
Multi-Compendial L-Arginine
Drug Master File (DMF) # CBER 19116 & CDER 38012
Chinese CDE # F20190000412
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
USP
EP
JP
ChP
Parenteral Formulation
Liquid Formulation
Pharmaceutical Process
Biopharma Process
L-Arginine (Plant Derived)
Highest – Parenteral Excipient Grade GMP
Multi-Compendial L-Arginine
Drug Master File (DMF) # CBER 19116 & CDER 38012
Chinese CDE # F20190000412
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
USP
EP
JP
ChP
Parenteral Formulation
Liquid Formulation
Pharmaceutical Process
Biopharma Process
100g, 1kg, 5kg, 10kg, 25kg, and 50kg