Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
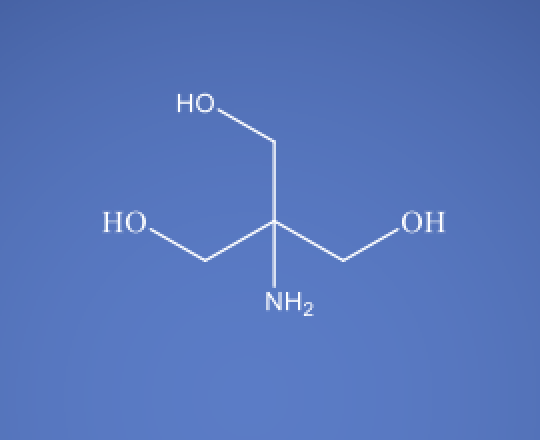
TRIS Base USP EP BP ChP
Molecular Formula
C4H11NO3
CAS No
Molecular Weight
Solubility in Water
Boiling Point
Melting Point
Quality Level
Highest – Parenteral Excipient Grade cGMP
Multi-Compendial Tris Base
Suitable for Injectable Biologic & Vaccine Formulations
Tris Base is a commonly used buffer in biologics production and various biochemical and molecular biology applications. It is a zwitterionic amine, meaning it has both acidic and basic functional groups in its structure, making it an effective buffer over a wide pH range.
TRIS Base is known for its ability to maintain a stable pH over a broad range. Typically, TRIS Base pH is between 7 and 9. This is crucial in biologics production processes where maintaining a specific pH is essential for the stability and functionality of proteins.
Pfanstiehl Multi-Compendial TRIS Base is relatively biologically inert, which is important when working with sensitive biological molecules. It minimizes the potential for interference with biochemical reactions or interactions.
(TRIS Base Molecular Weight 121.14 g/mol, Solubility: 670 mg/ml in water) is highly soluble in water, making it easy to prepare stock solutions for use in various applications. This solubility is essential in biologics production where precise and reproducible conditions are necessary.
Pfanstiehl Mult-Compendial TRIS Base exhibits low ultraviolet (UV) absorbance, which is important when working with biomolecules that are sensitive to UV light. Low absorbance helps prevent interference with spectrophotometric measurements.
Pfanstiehl TRIS Base has a good buffering capacity, allowing it to resist changes in pH when small amounts of acid or base are added. This is critical in maintaining a stable environment for biologics during various stages of production.
TRIS Base is generally considered non-toxic at typical working concentrations, which is crucial for applications involving cells or proteins where cytotoxicity could be a concern.
TRIS Base is versatile and can be used in a variety of applications, including electrophoresis, protein purification, enzyme assays, and other molecular biology techniques.
Pfanstiehl Brand High Purity – Low Endotoxin – Low Metals TRIS Base
was developed specifically for biopharmaceutical manufacturing and is produced under full ICH-Q7 cGMP conditions in the United States to the highest quality and purity standards.
Pfanstiehl Brand Multi-Compendial TRIS Base is compliant with USP EP BP and ChP pharmocopoeia.
Because of these stringent manufacturing specifications and Pfanstiehl quality systems, you can be assured that Pfanstiehl’s Tris Base will be of the highest quality & consistency from batch to batch and the safest available for use in your manufacturing or research application.
TRIS Base (Synthetic)
Highest – Compendial Parenteral Excipient Grade GMP
Multi-Compendial Tris Base
Suitable for Biologics or mRNA vaccine Final Formulation
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
USP
EP
BP
ChP
Parenteral Formulation
Liquid Formulation
Pharmaceutical Buffering
Pharmaceutical Process
Biopharma Process
TRIS Base (Synthetic)
Highest – Compendial Parenteral Excipient Grade GMP
Multi-Compendial Tris Base
Suitable for Biologics or mRNA vaccine Final Formulation
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
USP
EP
BP
ChP
Parenteral Formulation
Liquid Formulation
Pharmaceutical Buffering
Pharmaceutical Process
Biopharma Process
100g, 1kg, 5kg, 10kg, 25kg, and 50kg