Pfanstiehl Sucrose Citation Page
Menu
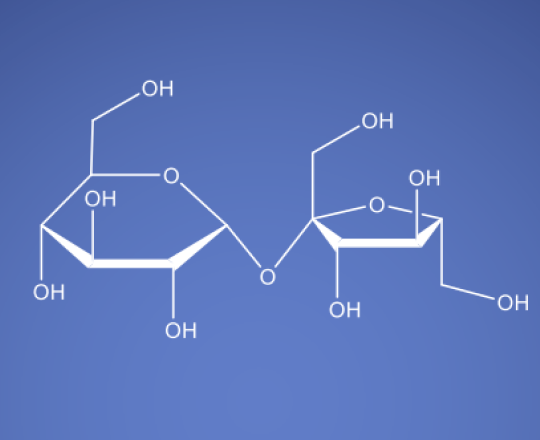
Molecular Formula
C12H22O11
CAS No
Molecular Weight
Solubility in Water
Boiling Point
Melting Point
Quality Level
Highest – Parenteral Excipient Grade – GMP / ICH-Q7
Suitable for Injectable Biologic & Vaccine Formulations
DMF # Yes – See Below
Chinese CDE # Yes – See Below
Sucrose (saccharose) is a non-reducing crystalline disaccharide made up of glucose and fructose, found in many plants but extracted as ordinary sugar mainly from sugar cane and sugar beets.
Multi-compendial Sucrose Excipient by Pfanstiehl is a high-purity, low endotoxin, low metals excipient that meets the stringent quality and regulatory requirements outlined in multiple pharmacopeias (e.g., USP, EP, JP). It is specifically manufactured and tested to ensure it is suitable for use in parenteral (injectable) drug products, where safety, sterility, and low impurity levels are critical.
Pfanstiehl High Purity – Low Endotoxin Sucrose is used in the biopharmaceutical industry to stabilize proteins, lipids, and carbohydrates throughout the formulation and freeze/thaw life cycle of therapeutics. It is also widely applied as a cryopreservative and media supplement in a variety of cell-based bioprocesses.
One of the key applications of low endotoxin sucrose (molecular weight 342.3 g/mol) lies in stabilizing proteins and antibodies. Proteins are inherently sensitive to environmental factors such as temperature, pH, and agitation, which can lead to denaturation and loss of biological activity. Sucrose (saccharose) acts as a stabilizing agent by forming hydrogen bonds with proteins, creating a protective environment that shields them from external stressors. This stabilizing effect helps maintain the native conformation of proteins and antibodies, preserving their functionality over time.
Pfanstiehl Multi-Compendial Sucrose Excipient Grade is widely employed in the stabilization of vaccines, where maintaining the integrity of antigens is crucial for effective immunization. Vaccines often face challenges during storage, transportation, and distribution, including exposure to temperature variations. The addition of pharmaceutical grade sucrose to vaccine formulations creates a glassy matrix that reduces molecular mobility, preventing the denaturation of antigens. This stabilizing effect ensures that vaccines remain potent and efficacious, even under challenging conditions.
Lipids, essential components of cell membranes and carriers for drug delivery, are prone to oxidation and degradation. By forming a protective barrier around lipid structures, it prevents the formation of free radicals, preserving the integrity and functionality of lipids. This stabilization is crucial for the development of lipid-based drug delivery systems and other pharmaceutical applications.
Pfanstiehl Low Endotoxin Sucrose is an effective cryopreservative agent, enabling the preservation of biological materials at low temperatures. In cryopreservation, the carbohydrate acts as a cryoprotectant by reducing the formation of ice crystals that can damage cells. Its ability to maintain a glassy state during freezing protects cellular structures and prevents the detrimental effects of ice formation, allowing for the long-term storage of cells, tissues, and biological samples.
In the field of cell therapy, Pfanstiehl pharmaceutical grade sucrose plays a pivotal role in preserving the viability and functionality of stem cells. Stem cells are highly sensitive to changes in their microenvironment, and sucrose helps maintain a stable environment during the freezing and thawing processes. This ensures the successful preservation of stem cells, allowing for their use in various therapeutic applications without compromising their regenerative potential.
Pfanstiehl Sucrose Citation Page
Pfanstiehl Sucrose High Purity – Low Endotoxin – Low Metals (HPLE-LM™) was developed specifically for biopharmaceutical formulation and commercial manufacturing and is produced under full ICH-Q7 cGMP conditions in the United States to the highest quality and purity standards.
This product is compliant with NF (National Formulary) EP (European) JP (Japanese) and ChP (Chinese) Pharmacopoeia. Pfanstiehl also holds a DMF for the S-124-2-MC product.
Because of these stringent manufacturing specifications and Pfanstiehl Quality Systems, you can be assured that Pfanstiehl products will be of the highest quality & consistency from batch to batch and the safest available for use in your manufacturing or research application.
Sucrose (Plant Derived)
Parenteral Excipient Grade – GMP (ICH-Q7)
Injectable Excipient Grade – cGMP
Multi-Compendial NF EP JP ChP
Drug Master File # 19538
Chinese CDE # F20170000125
Drug Master File # 37676
Chinese CDE # F20180001457
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
Lowest Nitrosamines
NF
EP
BP
JP
ChP
Parenteral Biologic Formulation
Liquid Formulation
Pharmaceutical Process
Cryopreservation
Lyophilization
Sucrose (Plant Derived)
Parenteral Excipient Grade – GMP (ICH-Q7)
Injectable Excipient Grade – cGMP
Multi-Compendial NF EP JP ChP
Drug Master File # 19538
Chinese CDE # F20170000125
Drug Master File # 37676
Chinese CDE # F20180001457
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
Lowest Nitrosamines
NF
EP
BP
JP
ChP
Parenteral Biologic Formulation
Liquid Formulation
Pharmaceutical Process
Cryopreservation
Lyophilization
100g, 1kg, 5kg, 10kg, 25kg, and 50kg