Pfanstiehl Histidine Citation Page
Menu
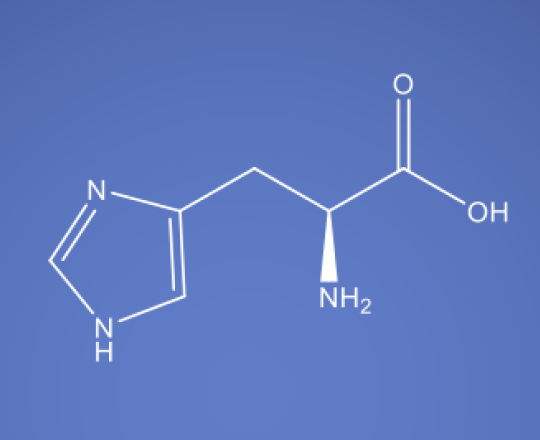
Molecular Formula
C6H9N3O2
CAS No
Molecular Weight
Solubility in Water
Boiling Point
Melting Point
Quality Level
Highest – Parenteral Excipient Grade cGMP
Suitable for Injectable Biologic & Vaccine Formulations
Drug Master File (DMF) # CDER 38409
Chinese CDE # F20200000338
L-Histidine is an essential amino acid that plays a crucial role in various biological processes within the human body. The amino acid has garnered attention in the pharmaceutical industry for its unique ability to enhance the stability of monoclonal antibodies (mAbs) during the manufacturing processes.
Monoclonal antibodies and other protein based biologic therapeutics are inherently susceptible to degradation, aggregation, and loss of activity during manufacturing and storage. L-Histidine emerges as a key player in addressing these challenges due to its unique chemical properties.
L-Histidine exhibits an excellent buffering capacity, particularly at pH 7.4, making it an ideal amino acid for maintaining a stable environment during the production and storage of mAbs. The pH level is a critical factor influencing the stability and solubility of proteins. By utilizing the amino acid at pH 7.4, manufacturers create an environment that closely mimics the physiological conditions within the human body, ensuring the stability of mAbs.
Pfanstiehl Multi-Compendial L-Histidine possesses metal-chelating properties, which can be advantageous in preventing metal-catalyzed oxidation and degradation of mAbs. Metal ions such as copper and iron can accelerate oxidative reactions, leading to the formation of reactive oxygen species. By chelating these ions, the amino acid acts as a protective agent, mitigating the risk of oxidative damage to mAbs.
The solubility of proteins is a critical parameter during manufacturing and formulation processes. L-Histidine, especially at pH 7.4, aids in maintaining the solubility of monoclonal antibodies, preventing aggregation and precipitation that could compromise the efficacy of the final product.
Pfanstiehl Histidine Citation Page
Pfanstiehl Brand High Purity – Low Endotoxin – Low Metals
were developed specifically for biopharmaceutical manufacturing and are produced under full ICH-Q7 cGMP conditions in the United States to the highest quality and purity standards.
Pfanstiehl Brand Multi-Compendial Amino Acids are compliant with multiple pharmacopoeia.
Because of these stringent manufacturing specifications and Pfanstiehl quality systems, you can be assured that Pfanstiehl products will be of the highest quality & consistency from batch to batch and the safest available for use in your manufacturing or research application.
L-Histidine (Plant Derived)
L-Histidine Inhalation Grade (Plant Derived)
Highest – Parenteral Excipient Grade GMP
Suitable for Injectable Biologic & Vaccine Formulations
Drug Master File (DMF) # CDER 38409
Chinese CDE # F20200000338
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
USP
EP
BP
JP
ChP
Parenteral Formulation
Liquid Formulation
Pharmaceutical Process
Biopharma Process
L-Histidine (Plant Derived)
L-Histidine Inhalation Grade (Plant Derived)
Highest – Parenteral Excipient Grade GMP
Suitable for Injectable Biologic & Vaccine Formulations
Drug Master File (DMF) # CDER 38409
Chinese CDE # F20200000338
Highest Purity
Lowest Endotoxin
Lowest Metals
Lowest Bioburden
USP
EP
BP
JP
ChP
Parenteral Formulation
Liquid Formulation
Pharmaceutical Process
Biopharma Process
100g, 1kg, 5kg, 10kg, 25kg, and 50kg